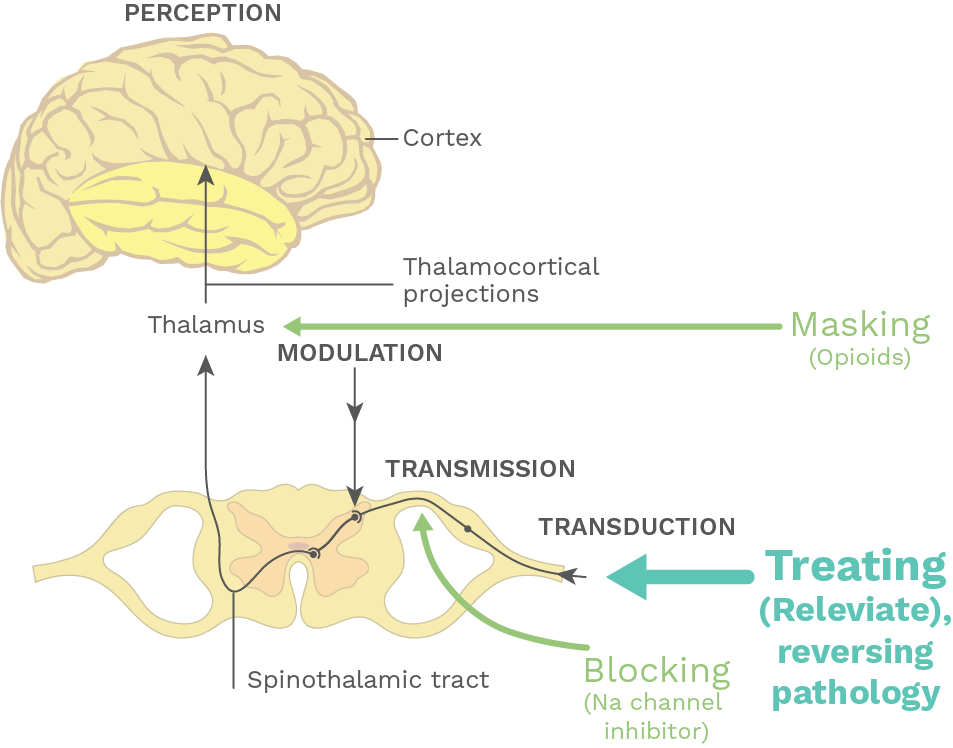
Developing disease-modifying neuropathic pain therapies that reverse pathophysiology, relieve chronic pain, and potentially cure it
Targeting Pain at Its Source
Releviate’s therapy inactivates secreted Matrix Metalloproteases that cause chronic pain.
Approach is very similar to the Abbvie’s Humira: inactivating the master-switch (in the case of Humira – TNF), which affects multiple pathophysiological pathways.
Instead of masking or blocking the pain pathway, Releviate’s therapy treats the underlying pathology resulting in painful sensation.
The Cost of Pain
Chronic pain is the largest indication in the world and the #1 reason for doctors visits in the U.S. Approximately 100 million adults in the U.S. are affected by chronic pain each year. The annual total cost of pain – including direct costs, decreased wages, and lost productivity – eclipses that of any other condition.
Addressing Unmet Needs in Chronic Pain
There is significant pre-clinical evidence that Releviate’s therapy is disease-modifying, reversing underlying pathophysiology leading to the neuropathic pain onset and maintenance. Based on this new insight into mechanisms of neuropathic pain, a Releviate-associated team developed patented, human monoclonal antibodies that inactivate MMP-9 and MMP-14.
READ MORE
Releviate holds an exclusive license for the technology. READ MORE